28 Mar 2024
University spinout Amber Therapeutics reports positive preliminary results from first-in-human study of Amber-UI
Amber Therapeutics, a leading medical technology company, has announced encouraging preliminary results from its first-in-human investigation of Amber-UI, a groundbreaking implanted pudendal neuromodulation system designed to address urinary incontinence
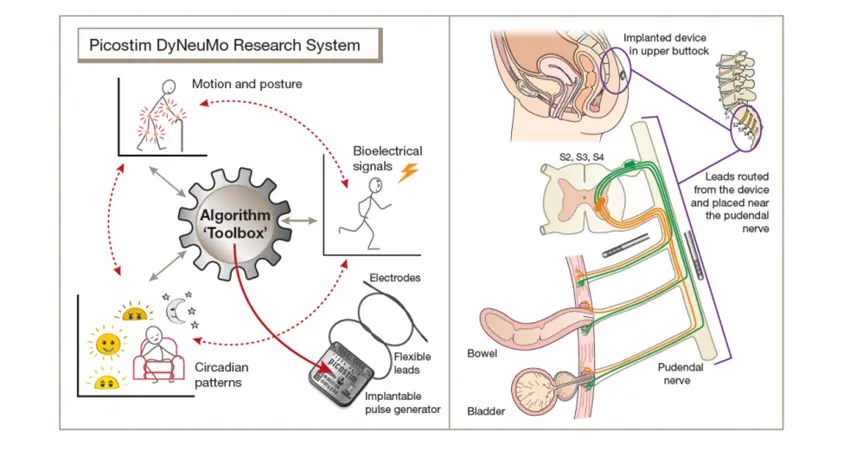
The Picostim-DyNeuMo research system toolbox was developed as a general-purpose bioelectronic therapy development platform. For the AURA-2 study, leads were placed along the pudendal nerve and the implanted system provides stimulation patterns under control of the research participant
The preliminary findings from the AURA-2 study have demonstrated the safety and scalability of Amber-UI therapy, showcasing its potential as an effective treatment option. Key results from the study showed that 80% (4 of 5) of women with mixed urinary experienced complete resolution of incontinence episodes six months after the implantation. These results signify a significant step forward in helping patients regain control over their bladder function and improve their quality of life. The pilot results were shared at the annual meeting of the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction, a leading international meeting for incontinence treatment.
The study, conducted at the University Hospital Antwerp in Belgium, involved 13 women with severe refractory urge urinary incontinence (a strong and sudden urge to urinate that is difficult to control or manage effectively with existing treatment options), or mixed urinary incontinence. The mixed urinary urge sub-group is the long-term focus of the Amber-UI therapy.
Amber-UI represents a major advancement in the field of urinary incontinence treatment. It is the first fully implantable closed-loop bioelectrical therapy that can access and target the pudendal nerve, which plays a crucial role in controlling continence. The therapy is capable of regulating the urge to void the bladder and increasing resistance to urine leakage caused by activities such as coughing or lifting, helping to restore normal bladder function.
Professor Tim Denison is Chief Engineer of Amber Therapeutics and RAEng Chair in Emerging Technology at the Department of Engineering Science (Institute of Biomedical Engineering) and Nuffield Department of Clinical Neurosciences, University of Oxford. He says, “the AURA-2 study leveraged the Picostim DyNeuMo research platform, which is a collaboration between academic and industry partners to enable clinical neuroscience and therapy translation. The Picostim-DyNeuMo specifically enables adaptive therapies to be efficiently prototyped and evaluated in first-in-human studies”.
In addition to demonstrating the safety and feasibility of Amber's novel surgical procedure, which accurately implants two electrode leads on the pudendal nerve, the AURA-2 study also tested adaptive algorithms using Amber-UI’s embedded sensors and classifiers. The sensors collect valuable information about the inertial (e.g. shock) and electrical activity in the body and the functioning of the urinary system that can be used to constantly adjust the therapy in response to the specific needs of each patient. This adaptive approach will be further refined in the next trial.
Lead investigator for the AURA-2 study, Dr. Stefan De Wachter, Professor of Urology at Antwerp University and co-founder of Amber Therapeutics, expressed his excitement about the preliminary results. He emphasized the potential of Amber-UI to be a safe and scalable solution for patients, offering personalized therapy tailored to their needs. This breakthrough technology has the potential to significantly improve the lives of millions of people suffering from urinary incontinence worldwide. By providing a personalized and effective treatment option, Amber-UI could offer new hope and restore bladder control for those affected by this common and debilitating condition.
About Amber Therapeutics
Amber Therapeutics is an innovative medical technology company focused on developing next-generation bioelectrical therapies. Their mission is to transform clinical outcomes for patients with functional disorders of the peripheral nervous system. The Picostim-DyNeuMo research platform is the result of a unique public-private partnership to create medical devices enabling translational research and manufactured in the UK. Amber Therapeutics was founded by Aidan Crawley (CEO, prior entrepreneur in residence at Oxford Science Enterprises), Professor Tim Denison (CE), Professor Charlie Knowles (QMUL), and Professor Stefan de Wachter (UZ Antwerp).




